HIV 1/2 Testing Algorithm
HTLV-III Antibodies, HIV Antibodies, HIV Ag/Ab Test, HIV Combo, HIV 1/2 Screen, HIV Screen
Test Codes
EPIC: LAB5766, HIV4G
Department
Chemistry
Specimen Collection Criteria
Collection Requirements:
- One dedicated gold-top serum separator tube (SST). This tube cannot be shared with any other test.
- The gold-top SST tube should be filled appropriately ( 5.0 mL); partially filled tubes may result in testing delays and possible requests for patient redraw.
- No other test can be added on to this dedicated tube.
If you have any questions, please contact Client Services (1-800-551-0488, option 5)
Physician Office/Draw Specimen Preparation
Let SST specimens clot 30-60 minutes then immediately centrifuge to separate serum from cells. Refrigerate (2-8°C or 36-46°F) the centrifuged SST tube within two hours of collection. (Minimum: 2.0 mL)
Preparation for Courier Transport
Transport: Centrifuged SST tube, refrigerated (2-8°C or 36-46°F). (Minimum: 2.0 mL)
Rejection Criteria
- Heat inactivated serum.
- Severely lipemic or hemolyzed specimens.
- Specimens that have obvious bacterial contamination.
- Red-top tubes with serum not separated from cells within two hours of collection.
In-Lab Processing
Let SST specimens clot 30-60 minutes. Centrifuge SST tubes or Microtainers® to separate serum from cells. Deliver immediately to the appropriate testing station.
Storage
Specimen Stability for Testing:
Centrifuged SST Tubes and Microtainers® with Separator Gel
Room Temperature (20-26°C or 68-78.8°F): 2-4 hours
Refrigerated (2-8°C or 36-46°F): 7 days
Frozen (-20°C/-4°F or below): Unacceptable
Red-top Tubes and Microtainers® without Separator Gel
Room Temperature (20-25°C or 68-77°F): 2-4 hours
Refrigerated (2-8°C or 36-46°F): Unacceptable
Frozen (-20°C/-4°F or below): Unacceptable
Serum Specimens (Pour-Overs)
Room Temperature (20-26°C or 68-78.8°F): 72 hours
Refrigerated (2-8°C or 36-46°F): 7 days
Frozen (-20°C/-4°F or below): 7 days
Specimen Storage in Department Prior to Disposal:
Refrigerated (2-8°C or 36-46°F): 7 days
Laboratory
Dearborn Chemistry Laboratory
Royal Oak Automated Chemistry Laboratory
Grosse Pointe Chemistry Laboratory
Troy Chemistry Laboratory
Farmington Hills Chemistry Laboratory
Performed
Sunday – Saturday, 24 hours a day.
Negative results available within 24 hours.
Positive results for HIV-1 Antibodies available within 24 hours.
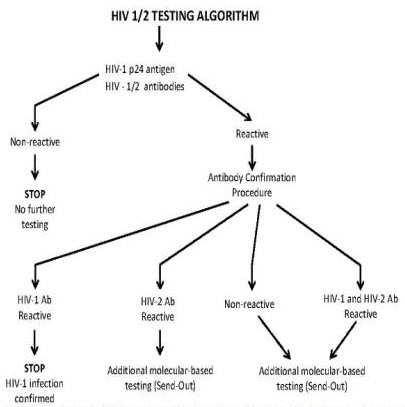
If send out testing for HIV-1 RNA or additional testing for HIV-2 is necessary, results may take an additional week. See algorithm below.
Reference Range
Negative.
Test Methodology
Chemiluminescence Immunoassay.
Interpretation
- A negative test result does not rule out the possibility of exposure to, or infection with, HIV.
- An initial positive screen will be followed by confirmation testing (see algorithm below) and an interpretative report summarizing results of all tests will be issued.
Clinical Utility
The initial screen is a 4th generation assay that detects both HIV-1 p24 antigen and antibodies to HIV-1 (groups M and O) and HIV-2. The new screen will detect acute HIV infection, on average 7 to 10 days earlier than the previously used antibody-only screen.
Positive screen confirmation antibody testing to distinguish HIV-1 from HIV-2 will be performed in-house within 24 hours of initial testing. Western blots (currently a send-out test) will no longer be ordered. Rarely, additional molecular-based confirmation testing for HIV-1 and/or HIV-2 will be performed (Send Out) as delineated in the algorithm below. All test results will be included in a single report with a final interpretation.
HIV-1 IgG is first detectable 3-12 weeks after infection in nearly all cases except neonates. Once established, HIV antibody levels usually persist throughout the lifetime of the patient. The presence of antibody does not imply immunity to the virus but rather, that the patient is assumed to be infected, and infectious. (3) Little is known about the antibody response to HIV-2 infection. The response is presumed to be similar to HIV-1. (3)
Clinical Disease
Primary HIV infections are symptomatic in 50-70% of patients. These patients present with a variety of symptoms including Influenza-like or mononucleosis-like illness to more severe neurological symptoms. These symptoms may persist for a few days or up to two months. Rapid progression to AIDS is seen in patients that experience a longer acute illness. Most patients however, remain asymptomatic for 1 to greater than 10 years before the clinical symptoms of AIDS present. (2)
Disease Reporting
This is a reportable infection and positive results will be reported to the Oakland County Health Department. In Michigan, both physicians and laboratories are responsible for reporting AIDS/HIV respectively. For more information on reportable diseases, contact the Epidemiology Department at (248) 551-4040.
Epidemiology
HIV-1 caused a worldwide pandemic of acquired immunodeficiency syndrome and AIDS-related complex. (1) In 1995, the World Health Organization estimated that 18 million adults and 1.5 million children are infected with HIV resulting in 4.5 million AIDS cases worldwide. (1)
Incubation Period
The median time from HIV infection to AIDS is 8-10 years. Homosexual men and some neonates may progress to AIDS more rapidly than other groups. (1)
Transmission
HIV-1 is transmitted by blood, blood products, and body fluids. Major modes of HIV-1 transmission include sexual intercourse, parenteral transmission through shared or inadequately sterilized needles, transfusion of HIV-1 infected blood and blood factor concentrates, and mother-to-child transmission either in utero, at birth, or through breast feeding. (1)
Reference
- CDC. 2006. The global HIV/AIDS pandemic, 2006. MMWR. 55: 841-844.
- CDC. 2006. Epidemiology of HIV/AIDS---United States. 1981-2005. MMWR. 55: 589-592.
- Schusbach, J. 2003. Human Immunodeficiency viruses. Manual of Clinical Microbiology, 8th edition. P.R. Murray et al. (eds). ASM Press. Washington, D.C., pp. 1253-1281.
CPT Codes
Initial Screen: 87389.
If screen is positive: 86701, 86702.
Further confirmation testing (if necessary): 87535, 87538.
Contacts
Chemistry Laboratory – TR
248-964-8070
Name: Chemistry Laboratory – TR
Location:
Phone: 248-964-8070
Chemistry Laboratory – GP
313-473-1807
Name: Chemistry Laboratory – GP
Location:
Phone: 313-473-1807
Chemistry Laboratory – FH
947-521-5252
Name: Chemistry Laboratory – FH
Location:
Phone: 947-521-5252
Chemistry Laboratory – DBN
313-596-2196
Name: Chemistry Laboratory – DBN
Location:
Phone: 313-596-2196
Automated Chemistry Laboratory – RO
248-551-8065
Name: Automated Chemistry Laboratory – RO
Location:
Phone: 248-551-8065
Last Updated
4/16/2021
Microtainer® and Vacutainer® are registered trademarks of Becton, Dickinson and Company.
UroVysion® is a registered trademark of Abbott Laboratories. ThinPrep® is a registered trademark of Hologic, Incorporated.